CoEnzyme Roles vs Pharmacological Roles of Vitamin B12
Share
Spectrum Sciences is committed to creating the most effective Vitamin B12 Protocol available. Safety, effectiveness, and label transparency are at the heart of our transdermal product line.
In terms of how the protocol achieves a high level of effectiveness, this hinges not only on the CoEnzyme roles of Vitamin B12, but also upon the unique Pharmacological role of vitamin B12. In this post, we will distinguish these two roles and explain how dosing impacts the outcome we are hoping to achieve.
CoEnzyme Roles
The coenzymatic roles of vitamin B12 are well characterized in the available literature. These roles are what make vitamin B12 an indispensable nutrient in the human body.
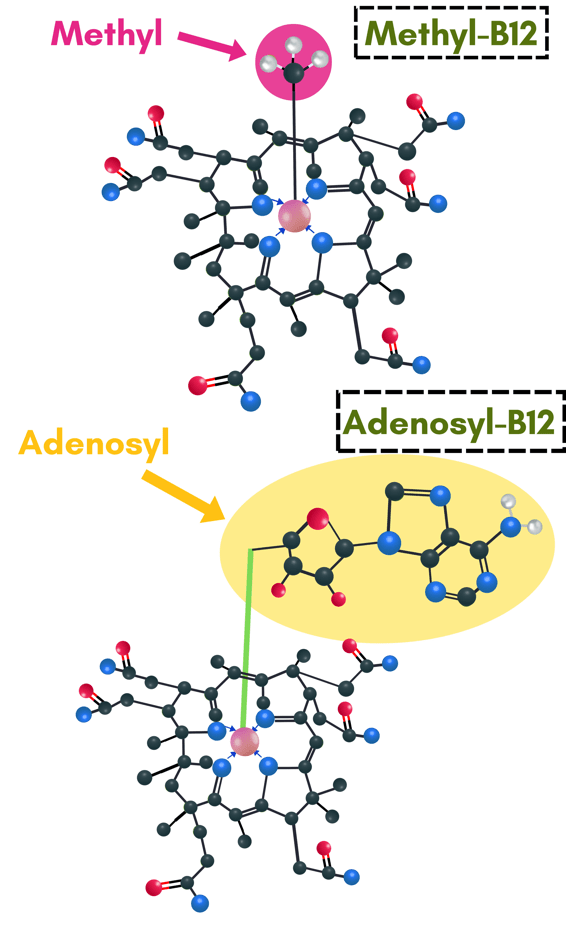
1) A cofactor for the enzyme methylmalonyl-CoA mutase.
2) A cofactor for the enzyme methionine synthase.
We will briefly touch upon what these enzymes do in the context of human metabolism.
Methylmalonyl-CoA mutase is a mitochondrial enzyme that uses adenosylcobalamin (also known as adenosyl-B12) as a cofactor in order to metabolize amino acids and odd-chain fatty acids for use in the Krebs cycle. The activity of this enzyme is directly involved in energy production.
Methionine synthase is a cytosolic enzyme that uses methylcobalamin (also known as methyl-B12) as a cofactor in order to metabolize homocysteine for continuation of the remethylation pathway. The activity of this enzyme can often be the limiting factor in maintaining robust methylation capacity.
Both of these coenzyme roles are dependent upon the body's availability of vitamin B12 AND the body's ability to activate vitamin B12 into these required cofactor forms. These enzymes do not require very much vitamin B12 to accomplish their duties, however, the activation of the available vitamin B12 pool is where these enzymes typically are hindered.
The first several steps of the B12 Protocol aim to address the issue of vitamin B12 activation, so that it can be put to use for energy production and methylation capacity maintenance.
The amount of vitamin B12 needed for these coenzyme roles is relatively tiny compared to the pharmacological role played by vitamin B12. If I had to estimate what percentage of an 8-drop dose of Activated B12 is utilized by the coenzyme roles, a generous estimate would be 2-3%.
Yes, it really might be that small. The RDA for vitamin B12, which is the amount a healthy human needs to operate methylmalonyl-CoA mutase and methionine synthase, is only about 6 micrograms.
Pharmacological Role
The remaining 97-98% of the applied vitamin B12 is fulfilling the pharmacological role of vitamin B12. Let's define how vitamin B12 operates in a pharmacological manner.
In the case of Vitamin B-12, when it is supplied at supraphysiological levels, it begins to take on a new role that was not previously characterized by the above cofactor roles. This is confirmed by laboratory testing specifically in the case of Vitamin B-12. Allow me to explain.
You see, if a person reports to the Emergency Department with symptoms of acute cyanide poisoning, the primary antidote is a megadose of Vitamin B-12 given by injection, repeated if necessary. This is not because methionine synthase or methylmalonyl-CoA mutase are involved in cyanide detoxification. This is because when given in supraphysiological amounts, Vitamin B-12 is able to directly bind to cyanide, neutralizing its toxic properties, while allowing it to be eliminated from the body.
Please understand that this use for Vitamin B-12 has absolutely nothing to do with its function in the body as a vitamin and does not begin to manifest until the concentration of the nutrient greatly exceeds what is needed to load the molecule onto the enzymes it serves in a cofactor capacity. This is why a massive pharmacological dose is required to observe the pharmacological role just described.
1. Okay, so you might be wondering: "What does acute cyanide poisoning have to do with conditions involving oxidative stress, such as autism, chronic fatigue syndrome, depression, anxiety, etc.?"
In 2000, a researcher from the University of Birmingham conducted a study that showed the autistic sample group was able to detoxify cyanide at only 12% the capacity of the neurotypical sample group (the enzyme involved in cyanide removal is called rhodanese). This is likely NOT a genetic short-coming of the autistic sample group, but rather a decrease in enzyme function as a result of oxidative stress in the mitochondria, which affects the function of MANY enzymes. Though the study group only included those who were diagnosed with autism spectrum disorders, this scenario, being a function of oxidative stress, likely applies to many other conditions that are characterized by a high load of oxidative stress.
Because direct cyanide exposure is very minute in the general population, this reduced rhodanese activity would not be expected to result in acute poisoning symptoms. Even with rhodanese working at only 12% capacity, the body can be expected to keep cyanide levels low enough to maintain vital functions, albeit with greater difficulty and loss of efficiency in the mitochondria. Because cyanide blocks oxidative phosphorylation within the mitochondria, energy production is greatly reduced and oxidative stress is even further increased. Over time, symptoms of mitochondrial dysfunction and if this situation occurs during crucial developmental stages of life, developmental delays would be expected if the situation is not corrected. If the situation begins after those crucial developmental stages have concluded, we can expect different disease states to manifest. The longer the damage is allowed to continue, the longer the road to recovery.
Vitamin B-12 therapy in conditions involving oxidative stress, I speculate, owes a great deal of its success to the same mechanism employed in Emergency Departments around the globe treating acute cyanide poisoning. The Vitamin B-12 molecule sacrifices itself to neutralize the toxic effects of cyanide within the mitochondrial electron transport chain, allowing the process of oxidative phosphorylation to take place. It is only with pharmacological doses of Vitamin B-12 that this mechanism will work.
An important distinction between chronic cyanide toxicity and acute cyanide poisoning must be made. To clarify this distinction, chronic toxicity usually involves long-term exposure to amounts of a toxicant that are not likely to be lethal in a short period of time (or ever) but can result in a deterioration of health if not addressed. Contrast that with acute poisoning, which is an immediately dangerous situation involving exposure to lethal levels of a toxicant over a short period of time, resulting in death or serious injury if immediate action is not taken.
We must acknowledge that in autism and other conditions involving high oxidative stress, the relationship with cyanide is a chronic toxicity situation – not an acute poisoning situation for which a visit to the Emergency Department is warranted – therefore the therapy for these conditions should look a bit different from the therapeutic process used in an acute poisoning situation.
The importance of each role
I would be remiss if I left the reader under the impression that only 2-3% of the benefits conferred upon the user come from the coenzymatic roles of vitamin B12 and the vast majority of the benefits are from the pharmacological role. We at Spectrum Sciences do not believe that is the case.
The coenzymatic roles are essential to life - without proper function of these enzymes, we could not live. The pharmacological role, however, is reducing the workload of an enzyme that keeps us alive. Both roles are important. It is impossible to place a relative importance upon these two distinct roles of vitamin B12 in the B12 Protocol.
What is helpful, perhaps, is understanding where the majority of the B12 is being directed. For those with lower rhodanese activity, the pharmacological effect plays an increasingly more significant role in their healing.
With this understanding, it can also help a practitioner understand why measures of their patient's methylation capacity and energy production are not improving commensurate to their patient's symptom resolution.
In the next post, we will look at the havoc that ensues when epigenetic regulation failure causes energy production to divert from neurological development.
